Book Appointment Now
Understanding Cervical Cancer

Cervical Cancer Guide
Introduction
Cervical cancer is a type of cancer that starts in the cells of the cervix—the lower, narrow part of the uterus (womb) that connects to the vagina. It occurs when abnormal cells in the cervix grow uncontrollably and can potentially spread to other parts of the body. The most common cause of cervical cancer is a long-lasting infection with certain types of human papillomavirus (HPV), which is a sexually transmitted virus.
The discovery that human papillomavirus (HPV) infection is the main cause of cervical cancer changed how we approach prevention. This led to the development of HPV vaccines and improved screening methods like HPV DNA testing. Studying cervical cancer is important because it remains a leading cause of cancer-related deaths among women globally. This is especially true in low- and middle-income countries where access to preventive measures and healthcare is often limited.
Statistics
Global Incidence and Mortality
According to the Global Cancer Observatory (GLOBOCAN) 2022 data:
- Incidence: Approximately 662,000 new cases of cervical cancer were diagnosed worldwide.
- Mortality: Around 348,000 deaths were attributed to cervical cancer globally.
- Ranking: Cervical cancer is the fourth most common cancer among women worldwide.
Key Disparities
- Geographical Variation:
- High-Income Countries:
- Lower incidence and mortality rates due to effective screening and vaccination programs.
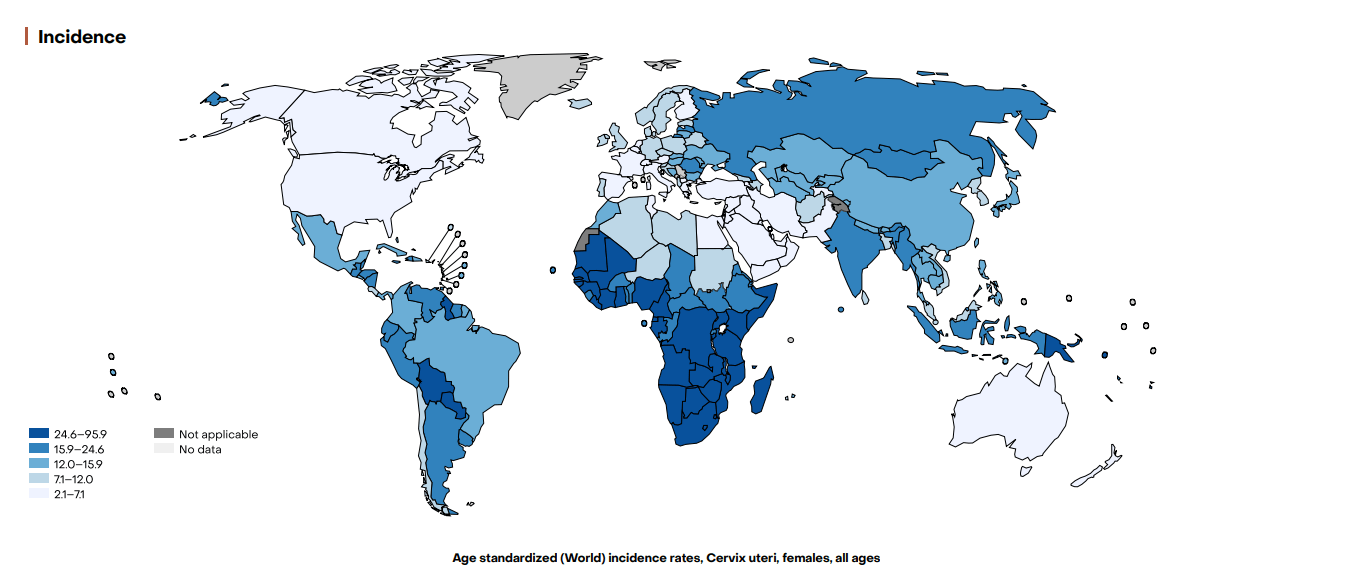
- Example: In North America, the incidence is about 6 per 100,000 women.
- Low- and Middle-Income Countries:
- Higher incidence and mortality rates, accounting for nearly 90% of global cases.
- Example: In sub-Saharan Africa, the incidence can be as high as 27 per 100,000 women.
- High-Income Countries:
- Age Distribution:
- Peak Incidence: Women aged 35 to 44 years.
- Mortality: Increases with age, with higher rates in women over 50 years.
- Socioeconomic Factors:
- Limited access to healthcare services.
- Lack of awareness and education about prevention and screening.
Key Statistics
- 5-Year Survival Rates (based on the stage at diagnosis):
- Stage I: Over 90%.
- Stage II: Approximately 60–80%.
- Stage III: Around 50%.
- Stage IV: Drops to 15–30%.
- Trends Over Time:
- Decreasing Incidence and Mortality:
- Observed in countries with established screening and vaccination programs.
- Example: A 70% reduction in incidence in developed countries over the past 50 years.
- Stable or Increasing Rates:
- In regions without adequate preventive measures.
- Example: Incidence remains high in parts of Africa, Southeast Asia, and Latin America.
- Decreasing Incidence and Mortality:
Cervical cancer incidence and rates in 2022

Source: International gco.iarc.who.int
Medical Illustrations
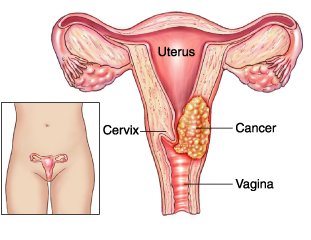
Cervix cancer:
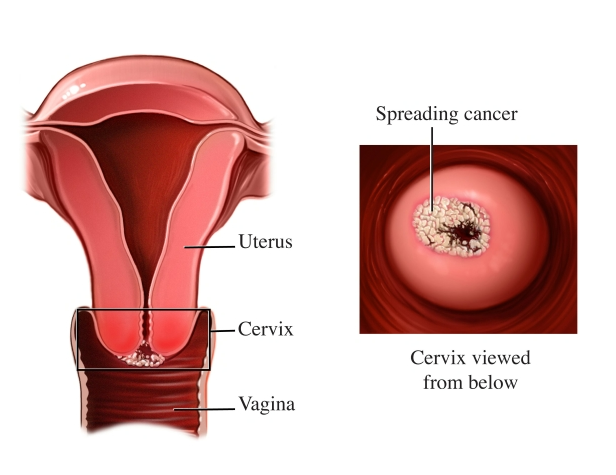
Cervix tumor:
Risk Factors and Prevention
a. Known Risk Factors
Human Papillomavirus (HPV) Infection:
- Primary Cause: High-risk HPV types, especially HPV 16 and 18, are responsible for about 70% of cervical cancers.
- Transmission: Predominantly through sexual contact.
Co-factors Enhancing Risk:
- Early Sexual Activity:
- Increases exposure risk to HPV.
- Multiple Sexual Partners:
- Higher likelihood of HPV infection.
- Smoking:
- Doubles the risk by impairing local immune response.
- Immunosuppression:
- HIV infection or immunosuppressive medications reduce the body’s ability to clear HPV.
- Long-term Oral Contraceptive Use:
- Slightly increases risk when used for over five years.
- High Parity:
- Having multiple full-term pregnancies is associated with increased risk.
Genetic Factors:
- Familial History:
- Slightly elevated risk if a first-degree relative had cervical cancer.
- Genetic Susceptibility:
- Variations in HLA genes affecting immune response to HPV.
Recent Studies:
- A meta-analysis in The Lancet Oncology (2021) reinforced the role of smoking as a significant co-factor.
- Research in International Journal of Cancer (2022) highlighted the interaction between HIV and HPV in accelerating cervical carcinogenesis.
b. Preventive Strategies
HPV Vaccination:
- Vaccines Available:
- Bivalent Vaccine: Targets HPV 16 and 18.
- Quadrivalent Vaccine: Targets HPV 6, 11, 16, and 18.
- Nonavalent Vaccine: Covers nine HPV types, including 31, 33, 45, 52, and 58.
- Efficacy:
- Over 90% effective in preventing infections with targeted HPV types.
- Recommendations:
- WHO: Vaccination for girls aged 9–14 years before sexual debut.
- CDC: Vaccination for both girls and boys starting at 11–12 years.
- Single-Dose Schedule:
- Emerging evidence suggests that a single dose may offer substantial protection.
- Study: Kang et al., 2021 showed comparable immune responses between single and multiple doses.
Screening Programs:
- Pap Smear (Cytology):
- Method: Microscopic examination of cervical cells.
- Frequency: Every 3 years starting at age 21.
- HPV DNA Testing:
- Method: Detects high-risk HPV types.
- Frequency: Every 5 years starting at age 30, often combined with cytology (co-testing).
- Advantage: Higher sensitivity than cytology alone.
- Visual Inspection with Acetic Acid (VIA):
- Method: Application of acetic acid to the cervix, observing for white areas.
- Usage: Resource-limited settings.
Safe Sexual Practices:
- Condom Use:
- Reduces HPV transmission, but does not eliminate risk entirely.
- Limiting Number of Sexual Partners:
- Decreases exposure likelihood.
Smoking Cessation:
- Reduces risk and improves overall cervical health.
HIV Management:
- Antiretroviral Therapy (ART):
- Improves immune function, aiding in HPV clearance.
Public Health Measures:
- Education Campaigns:
- Increase awareness about HPV and cervical cancer.
- Access to Care:
- Ensuring availability of vaccines and screening, especially in LMICs.
Screening
Current Screening Methods
Pap Smear (Cytology):
- Efficacy:
- Sensitivity: Approximately 50–75% for detecting high-grade lesions.
- Specificity: Around 98%, leading to few false positives.
- Advantages:
- Widely used and accepted.
- Cost-effective in organized programs.
- Limitations:
- Requires skilled cytotechnologists.
- Lower sensitivity compared to HPV testing.
HPV DNA Testing:
- Efficacy:
- Sensitivity: Over 90% for detecting high-grade lesions.
- Specificity: Approximately 86%.
- Advantages:
- Higher sensitivity allows for longer intervals between screenings.
- Can be performed on self-collected samples.
- Limitations:
- Higher initial cost.
- May detect transient infections that could resolve spontaneously.
Co-testing (Cytology + HPV Testing):
- Efficacy:
- Combines high sensitivity of HPV testing with high specificity of cytology.
- Recommendations:
- Every 5 years for women aged 30–65.
- Limitations:
- Increased cost and resource requirements.
Visual Inspection with Acetic Acid (VIA):
- Efficacy:
- Sensitivity: Around 77%.
- Specificity: Approximately 85%.
- Advantages:
- Low-cost, immediate results.
- Suitable for low-resource settings.
- Limitations:
- Operator-dependent.
- Lower accuracy compared to other methods.
Comparative Summary:
- HPV DNA Testing is preferred for its high sensitivity and ability to extend screening intervals.
- Pap Smear remains valuable, especially where HPV testing is not accessible.
VIA offers a practical alternative in resource-limited areas.
Symptoms and Signs
Early-Stage Symptoms:
- Often asymptomatic; underscores the importance of regular screening.
Intermediate-Stage Symptoms:
- Abnormal Vaginal Bleeding:
- Post-coital bleeding.
- Intermenstrual bleeding.
- Postmenopausal bleeding.
- Unusual Vaginal Discharge:
- Watery, bloody, or foul-smelling.
- Pelvic Pain:
- Especially during intercourse.
Advanced-Stage Symptoms:
- Lower Back or Leg Pain:
- Due to nerve or lymph node involvement.
- Swelling of the Legs:
- Lymphedema from lymphatic obstruction.
- Weight Loss and Fatigue:
- General signs of advanced cancer.
- Urinary or Rectal Symptoms:
- Hematuria or rectal bleeding if the tumor invades adjacent organs.
Misdiagnoses and Atypical Presentations:
- Case Anecdote:
- A 35-year-old woman with persistent vaginal discharge was treated repeatedly for infections before a Pap smear revealed high-grade lesions.
- Challenges:
- Symptoms overlap with benign conditions like infections or hormonal imbalances.
- Cultural stigma may prevent women from seeking timely medical attention.
Diagnosis Steps
- Initial Evaluation:
- Medical History:
- Assess symptoms, sexual history, and risk factors.
- Physical Examination:
- Pelvic exam to inspect the cervix for abnormalities.
- Medical History:
- Screening Tests:
- Pap Smear:
- Collection of cervical cells for cytological analysis.
- HPV DNA Test:
- Can be done simultaneously with Pap smear.
- Pap Smear:
- Colposcopy:
- Procedure:
- Visual examination of the cervix using a colposcope.
- Application of acetic acid or Lugol’s iodine to highlight abnormal areas.
- Indications:
- Abnormal Pap smear results.
- Suspicious lesions observed during pelvic exam.
- Procedure:
- Biopsy Techniques:
- Punch Biopsy:
- Small tissue samples taken from suspicious areas.
- Endocervical Curettage:
- Scraping cells from the cervical canal.
- Cone Biopsy (Conization):
- Removal of a cone-shaped section of tissue for more extensive analysis.
- Methods: Loop Electrosurgical Excision Procedure (LEEP) or cold knife conization.
- Punch Biopsy:
- Imaging Studies (for staging):
- MRI:
- Preferred for local tumor assessment and pelvic involvement.
- CT Scan:
- Evaluates lymph node involvement and distant metastasis.
- PET-CT Scan:
- Detects metabolic activity of cancer cells, useful for staging.
- MRI:
- Laboratory Tests:
- Complete Blood Count (CBC):
- Checks for anemia.
- Renal and Liver Function Tests:
- Assesses organ function before treatment.
- Complete Blood Count (CBC):
- FIGO Staging System:
- Stage I:
- Cancer confined to the cervix.
- Stage II:
- Spread beyond the cervix but not to the pelvic wall.
- Stage III:
- Extension to the pelvic wall or lower third of the vagina.
- Stage IV:
- Invasion into adjacent organs or distant metastasis.
- Stage I:
Stages
Types of Treatment
Treatment Modalities
Surgery:
- Conization:
- Indications: Early-stage cancer (Stage IA1).
- Procedure: Removal of a cone-shaped section of the cervix.
- Outcomes: Preserves fertility.
- Side Effects: Bleeding, infection, cervical stenosis.
- Hysterectomy:
- Types:
- Simple Hysterectomy: Removal of the uterus and cervix.
- Radical Hysterectomy: Includes removal of surrounding tissues (parametrium) and upper vagina.
- Indications: Early-stage cancers (Stage IA2 to IB1).
- Outcomes: High cure rates in early stages.
- Side Effects: Infertility, urinary dysfunction, sexual dysfunction.
- Types:
- Pelvic Exenteration:
- Indications: Recurrent cancer confined to the pelvis.
- Procedure: Extensive surgery removing pelvic organs.
- Side Effects: Significant impact on quality of life.
Radiation Therapy:
- External Beam Radiation Therapy (EBRT):
- Mechanism: High-energy X-rays to kill cancer cells.
- Indications: All stages, often combined with chemotherapy.
- Side Effects: Fatigue, skin irritation, gastrointestinal symptoms.
- Brachytherapy:
- Mechanism: Internal radiation by placing radioactive material close to the tumor.
- Advantages: Delivers high doses directly to the tumor.
- Side Effects: Vaginal stenosis, localized pain.
Chemotherapy:
- Agents:
- Cisplatin: Most commonly used.
- Carboplatin, Paclitaxel: Alternatives or in combination.
- Mechanism: Inhibits DNA replication in rapidly dividing cells.
- Indications:
- Concurrent with radiation in locally advanced stages.
- Palliative care in metastatic disease.
- Side Effects: Nausea, vomiting, nephrotoxicity, myelosuppression.
Targeted Therapy:
- Bevacizumab:
- Mechanism: Anti-angiogenesis by inhibiting vascular endothelial growth factor (VEGF).
- Indications: Advanced or recurrent cervical cancer.
- Outcomes: Improved median overall survival by 3.7 months.
- Side Effects: Hypertension, thromboembolic events, bleeding risks.
Immunotherapy:
- Pembrolizumab:
- Mechanism: PD-1 inhibitor enhancing immune response against tumor cells.
- Indications: PD-L1 positive recurrent or metastatic cervical cancer.
- Outcomes: Durable responses in a subset of patients.
Side Effects: Immune-mediated reactions (colitis, pneumonitis).
Evidence-Based Comparisons
| Treatment | Mechanism | Side Effects | Efficacy (Survival Rate) | Study/Trial |
|---|---|---|---|---|
| Chemoradiotherapy | DNA damage and inhibition of repair mechanisms | Fatigue, nausea, hematologic toxicity | Improved 5-year OS by ~12% over radiation alone | Keys et al., 1999 |
| Bevacizumab + Chemo | Inhibits angiogenesis (VEGF inhibitor) | Hypertension, bleeding, thromboembolism | Median OS improved by 3.7 months | GOG 240 Trial, 2014 |
| Pembrolizumab | PD-1 immune checkpoint inhibitor | Immune-mediated adverse events | Overall response rate of ~14% in PD-L1 positive patients | KEYNOTE-158, 2019 |
| HPV Vaccination | Stimulates immune response against HPV antigens | Injection site reactions, fever | Over 90% efficacy in preventing HPV 16/18 infections | PATRICIA Trial, 2009 |
Key Studies:
- GOG 240 Trial (2014):
- Findings: Bevacizumab combined with chemotherapy improved median overall survival from 13.3 to 16.8 months.
- Clinical Significance: Led to FDA approval of bevacizumab for advanced cervical cancer.
- KEYNOTE-158 (2019):
- Findings: Pembrolizumab showed an overall response rate of 14.3% in PD-L1 positive patients.
- Clinical Significance: Provided a new treatment option for recurrent or metastatic cervical cancer.
Additional Resources
- Patient Advocacy Groups:
- Key Opinion Leaders:
- Dr. Ted Trimble: Expert in gynecologic oncology and cervical cancer research.
- Dr. Rengaswamy Sankaranarayanan: Noted for work on cancer screening in LMICs
- Clinical Trials:
- ClinicalTrials.gov: Comprehensive database of ongoing cervical cancer studies.
- International Agency for Research on Cancer (IARC): Provides resources on global cancer statistics and research initiatives.
- Image sources:
Key Findings
- Cervical Cancer is Preventable: Through effective HPV vaccination and regular screening.
- HPV Infection is the Primary Cause: Emphasizes the importance of preventive strategies targeting HPV.
- Advancements in Screening: HPV DNA testing offers higher sensitivity and longer screening intervals.
- New Treatments Improve Survival: Targeted therapies and immunotherapies provide additional options for advanced disease.
- Global Disparities Exist: LMICs bear the highest burden due to limited access to prevention and treatment.
Discussion
The significant decline in cervical cancer incidence and mortality in high-income countries demonstrates the effectiveness of organized screening and vaccination programs. The strong causal link between HPV infection and cervical cancer provides a clear target for prevention efforts. HPV vaccines have shown remarkable efficacy and have the potential to drastically reduce the disease burden globally.
However, challenges remain, particularly in LMICs where the majority of cases occur. Factors such as limited healthcare infrastructure, cultural barriers, and economic constraints hinder the implementation of widespread vaccination and screening programs. The emerging evidence supporting single-dose vaccine schedules could simplify logistics and reduce costs, making vaccination more accessible.
Advancements in screening methods, particularly the shift towards primary HPV DNA testing, offer improved detection rates of precancerous lesions. Self-sampling techniques could enhance participation in populations with barriers to traditional screening methods.
Treatment options for advanced cervical cancer have expanded with the introduction of targeted therapies like bevacizumab and immunotherapies such as pembrolizumab. While these offer hope for improved outcomes, their high cost and limited availability in resource-poor settings pose significant challenges.
Addressing these disparities requires a multifaceted approach involving policy changes, international collaboration, and investment in healthcare infrastructure. Education and awareness campaigns are crucial to increase acceptance and uptake of preventive measures.
Final Recommendations
Clinical Recommendations:
- Vaccination:
- Implement HPV vaccination programs targeting girls (and boys) aged 9–14 years.
- Consider adopting single-dose schedules pending further evidence.
- Screening:
- Integrate HPV DNA testing into national screening guidelines.
- Utilize self-sampling methods to increase participation.
- Treatment:
- Incorporate new therapies like bevacizumab and pembrolizumab into treatment protocols where feasible.
- Ensure access to palliative care services.
Research Directions:
- Vaccine Optimization:
- Continue studies on single-dose efficacy and long-term protection.
- Develop vaccines covering more HPV types prevalent in different regions.
- Overcoming Treatment Resistance:
- Investigate molecular mechanisms underlying resistance to improve therapeutic strategies.
- Innovative Screening Methods:
- Develop cost-effective, point-of-care tests suitable for low-resource settings.
Policy Suggestions:
- Increase Funding:
- Allocate resources for vaccination and screening programs, especially in LMICs.
- International Collaboration:
- Support initiatives like the WHO’s Cervical Cancer Elimination Strategy.
- Education and Awareness:
- Implement culturally sensitive campaigns to promote preventive measures.
- Access to Affordable Treatments:
- Negotiate pricing and facilitate generic production to make therapies more accessible.
Disclaimer
The information provided in this article is intended for general informational purposes only and should not be construed as medical advice. While every effort has been made to ensure the accuracy of the information presented, it is not a substitute for professional medical guidance, diagnosis, or treatment. Always consult a qualified healthcare provider with any questions you may have regarding a medical condition, including cervical cancer. Do not disregard or delay seeking professional medical advice based on information found in this article. The authors and publishers are not responsible for any consequences resulting from the use of the information provided.
