Book Appointment Now
Demystifying Clinical Trials: What Participation Means for Patients with Metastatic Colorectal Cancer
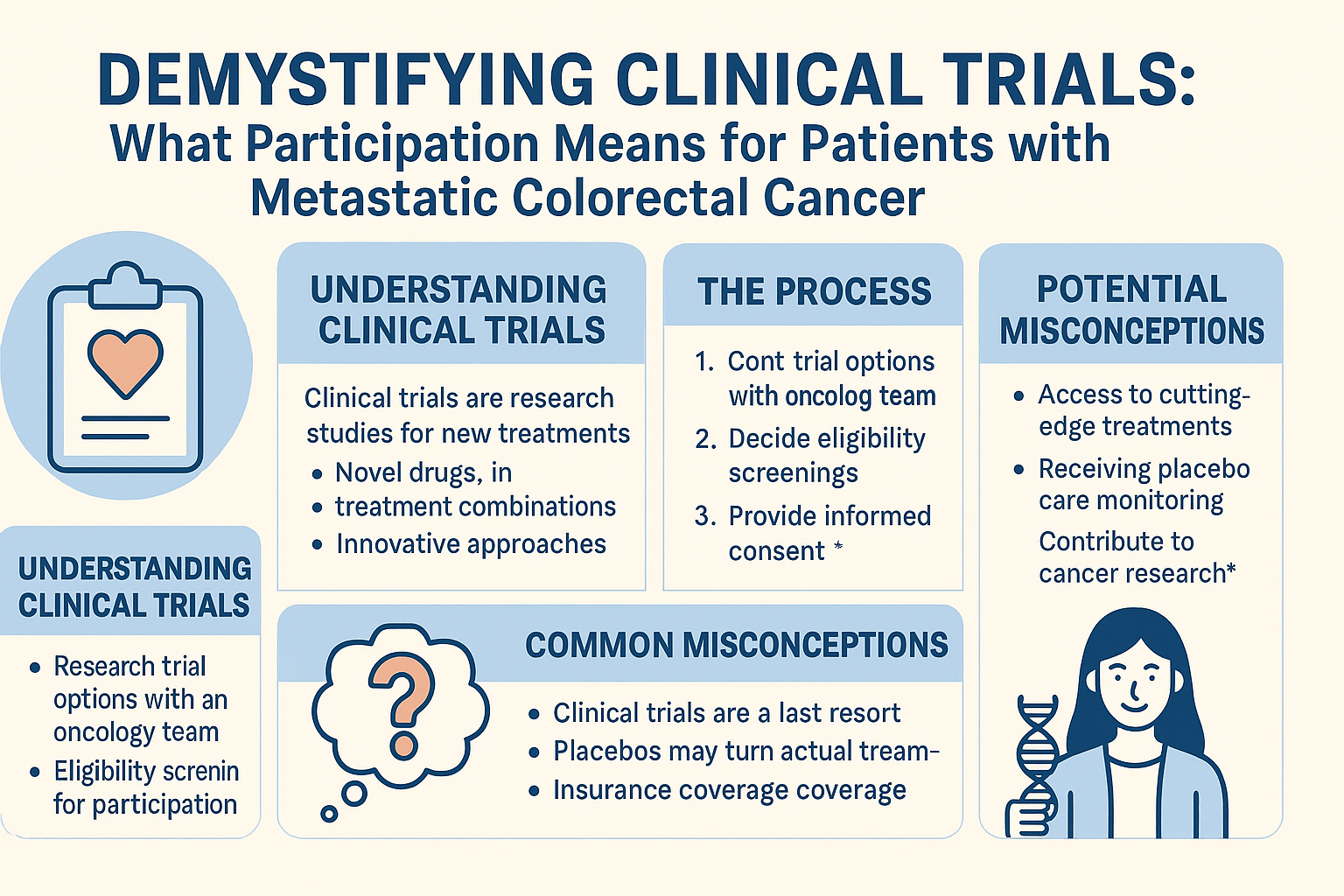
Introduction
For patients facing metastatic colorectal cancer (mCRC), the treatment landscape has evolved significantly over the past two decades. While standard treatments—surgery, chemotherapy, targeted therapies, and immunotherapy—have improved survival rates, many patients still experience disease progression and require additional treatment options. Clinical trials represent a crucial pathway for accessing novel approaches that may offer benefit when standard treatments have been exhausted or as alternatives to current standards of care.
Despite their importance, clinical trials remain misunderstood by many patients and families. Common misconceptions, concerns about receiving a placebo, confusion about the process, and uncertainty about insurance coverage can prevent eligible patients from considering trial participation.
This article aims to demystify clinical trials specifically for patients with metastatic colorectal cancer, providing clear information about the process, addressing common concerns, and highlighting potential benefits.
Understanding Clinical Trials in Metastatic Colorectal Cancer
What Are Clinical Trials?
Clinical trials are carefully designed research studies that evaluate new medical approaches in human participants. These studies are essential for advancing cancer care, as they determine whether new treatments are safe, effective, and superior to existing standards of care. For metastatic colorectal cancer specifically, clinical trials investigate:
- Novel therapeutic agents (new drugs or biologics)
- New combinations of existing treatments
- Innovative treatment approaches (e.g., immunotherapy, targeted therapy)
- Improved methods for managing symptoms and side effects
- Enhanced strategies for monitoring disease
- Interventions to improve quality of life during treatment
Types of Clinical Trials Relevant to Metastatic Colorectal Cancer
Phase I Trials
- Primary goal: Determine safety and appropriate dosing of new treatments
- Typical size: Small (15-30 participants)
- For mCRC patients: Often available to those whose cancer has progressed after multiple lines of standard therapy
- Current examples: Novel immunotherapy combinations, first-in-human studies of targeted agents for specific molecular alterations
Phase II Trials
- Primary goal: Assess whether the treatment shows activity against the cancer
- Typical size: Medium (50-100 participants)
- For mCRC patients: May be available after first-line or second-line treatment failure
- Current examples: Combination approaches with immune checkpoint inhibitors for microsatellite stable (MSS) tumors, novel targeted therapies for specific genetic alterations
Phase III Trials
- Primary goal: Compare new treatment to current standard of care
- Typical size: Large (hundreds to thousands of participants)
- For mCRC patients: Often available as first-line or second-line treatment options
- Current examples: New chemotherapy backbones, maintenance therapy strategies, immune therapy combinations for specific biomarker groups
Basket Trials
- Design: Enroll patients with different cancer types who share specific molecular characteristics
- For mCRC patients: Particularly relevant for those with less common genetic alterations (HER2 amplification, NTRK fusions, etc.)
- Current examples: Precision medicine approaches targeting specific mutations regardless of primary tumor site
Umbrella Trials
- Design: Test multiple targeted therapies in different subgroups of patients with the same cancer type
- For mCRC patients: Allow personalized treatment assignment based on molecular profiling
- Current examples: Platform trials testing multiple novel agents in biomarker-defined populations

The Clinical Trial Process for Metastatic Colorectal Cancer Patients
Finding Appropriate Trials
Identifying suitable clinical trials involves several steps:
- Comprehensive molecular profiling: Modern trials often require specific biomarker testing to determine eligibility. For mCRC patients, this typically includes:
- MSI/MMR status
- RAS mutation status (KRAS, NRAS)
- BRAF mutation status
- HER2 amplification
- NTRK fusions
- Tumor mutational burden (TMB)
- Discussing options with your oncologist: Your treatment team can suggest trials based on your specific situation, including:
- Previous treatments received
- Current performance status
- Molecular characteristics of your tumor
- Treatment goals and preferences
- Utilizing trial matching resources:
- ClinicalTrials.gov (searchable database of all registered trials)
- Fight Colorectal Cancer’s Trial Finder (colorectal cancer-specific)
- Colorectal Cancer Alliance’s Clinical Trial Finder
- Cancer centers’ clinical trial navigators
- Patient advocacy organizations’ trial navigation services
The Screening Process
Before enrolling in a trial, patients must complete screening procedures to confirm eligibility:
- Initial screening: Review of medical history, current medications, and previous treatments
- Physical examination: Assessment of overall health and performance status
- Laboratory tests: Blood work to evaluate organ function and blood counts
- Tumor assessment: Scans to document current disease status and establish baseline measurements
- Specialized testing: Molecular profiling if not already completed or additional biomarker tests specific to the trial
- Review of eligibility criteria: Confirmation that all inclusion criteria are met and no exclusion criteria apply
For metastatic colorectal cancer trials, common eligibility criteria include:
- Specific biomarker status (e.g., MSI-high, BRAF-mutated)
- Adequate organ function (liver, kidneys, bone marrow)
- Specific previous treatment history
- Performance status requirements (ability to perform daily activities)
- Absence of certain comorbidities
Informed Consent Process
Before participating in any clinical trial, patients must provide informed consent, which involves:
- Receiving detailed information about:
- Trial purpose and design
- Required procedures and visits
- Potential risks and benefits
- Alternatives to participation
- Rights and responsibilities
- Reviewing the informed consent document: This comprehensive document outlines all aspects of the trial in detail.
- Discussion with the research team: Opportunity to ask questions and address concerns.
- Voluntary decision-making: Patients should never feel pressured to participate.
- Ongoing consent: Participants can withdraw from a trial at any time without negative consequences for their care.
Participation Experience
What to expect during clinical trial participation:
Long-term follow-up after treatment completion in many trials
- Intravenous therapies at specified intervals
- Oral medications taken on a defined schedule
- Radiation therapy or surgical procedures
- Combination approaches
- More frequent clinic visits than standard care
- Additional blood tests and imaging studies
- Detailed symptom assessments
- Quality of life questionnaires
- Proactive monitoring for expected and unexpected side effects
- Clear guidelines for reporting problems
- 24/7 access to the research team for urgent issues
- Predefined criteria for dose modifications or treatment discontinuation
- Varies by trial design
- Typically continues until disease progression, unacceptable toxicity, or completion of the planned treatment course
- Long-term follow-up after treatment completion in many trials
Common Misconceptions About Clinical Trials for Metastatic Colorectal Cancer
Misconception #1: “Clinical trials are a last resort.”
Reality: While trials can provide options when standard treatments fail, they may also be appropriate earlier in treatment. For metastatic colorectal cancer, trials may be considered:
- As first-line treatment, especially for patients with specific molecular alterations
- After failure of first-line therapy
- In the maintenance setting after response to initial therapy
- For specific molecular subgroups at any point in treatment
Misconception #2: “I might receive a placebo and get no treatment at all.”
Reality: In metastatic colorectal cancer trials:
- Pure placebo-only arms are extremely rare
- If a placebo is used, it’s typically given alongside standard-of-care treatment
- Phase III trials may compare standard therapy to standard therapy plus a new agent
- Patients are always informed if a trial includes a placebo component
Example: A trial might compare FOLFOX chemotherapy + placebo versus FOLFOX + an investigational agent, ensuring all patients receive active treatment.
Misconception #3: “Insurance won’t cover clinical trial participation.”
Reality:
- The Affordable Care Act requires most health insurance plans to cover routine patient care costs in clinical trials
- Medicare covers routine costs in qualifying clinical trials
- Many state laws provide additional protections for clinical trial coverage
- Trial sponsors typically cover research-specific procedures and the investigational treatment itself
Misconception #4: “I’m too old for a clinical trial.”
Reality: Many clinical trials for metastatic colorectal cancer specifically seek to include older adults, as they represent a significant proportion of patients. Age alone is rarely an exclusion criterion, with functional status and organ function being more relevant factors.
Misconception #5: “Participating means becoming a ‘guinea pig.'”
Reality: Modern clinical trials:
- Prioritize patient welfare above research objectives
- Build upon substantial preclinical and early clinical evidence
- Follow rigorous protocols approved by regulatory bodies and ethics committees
- Include extensive safety monitoring and predefined stopping rules
Potential Benefits of Clinical Trial Participation for Metastatic Colorectal Cancer Patients
Access to Novel Treatments
Participants may gain access to therapies that are:
- Years away from FDA approval
- Targeting specific molecular alterations in their tumor
- Employing innovative mechanisms of action
- Not otherwise available outside a trial setting
Current examples in mCRC include:
- Bispecific antibodies targeting multiple pathways simultaneously
- Novel immunotherapy approaches for microsatellite stable tumors
- Targeted therapies for newly identified genetic alterations
- Antibody-drug conjugates with enhanced tumor specificity
Enhanced Monitoring and Care
Trial participants typically receive:
- More frequent clinical assessments
- More comprehensive imaging and laboratory testing
- Closer symptom monitoring
- Multidisciplinary team attention
- Additional supportive care resources
Advancing Cancer Research
Participation contributes to:
- Development of new treatment options for future patients
- Better understanding of colorectal cancer biology
- Identification of predictive biomarkers
- Improved management of treatment-related side effects
Financial Considerations
Potential financial benefits include:
- Possible reduction in out-of-pocket expenses for care
- Coverage of investigational treatment costs by trial sponsors
- Coverage of many standard care procedures by insurance
- Potential reimbursement for travel and lodging in some trials
Special Considerations for Metastatic Colorectal Cancer Trials
Biomarker Testing Requirements
The increasing precision of colorectal cancer treatment means that comprehensive biomarker testing is often required before trial enrollment. Important biomarkers include:
- MSI/MMR Status: Determines eligibility for immunotherapy trials
- RAS Mutations: Affects eligibility for EGFR-targeted therapy trials
- BRAF Mutations: Qualifies patients for BRAF-targeted approaches
- HER2 Amplification: Required for HER2-targeted therapy trials
- NTRK Fusions: Rare but targetable alteration in some trials
Treatment Line Considerations
Trials are available at different treatment stages:
- First-line trials: May require no prior systemic therapy for metastatic disease
- Second-line trials: Typically require progression on first-line treatment
- Third-line and beyond: Available for heavily pretreated patients
- Maintenance trials: Focus on treatment approaches after initial response
Quality of Life Assessment
Many modern mCRC trials incorporate:
- Patient-reported outcome measures
- Quality of life questionnaires
- Symptom burden assessments
- Functional status evaluation
- Financial toxicity monitoring
These components recognize that survival is not the only important outcome—how patients feel during treatment matters significantly.
Questions to Ask When Considering a Clinical Trial
About the Trial Design
- What is the main purpose of this clinical trial?
- What phase is this trial, and what does that mean for me?
- How many patients have received this treatment so far?
- Is there a chance I would receive a placebo?
- What are the treatment arms, and how are patients assigned to them?
- How does this experimental treatment differ from standard care?
About Practical Considerations
- Where will I receive treatment, and how often?
- What tests and procedures will be required?
- How long will I participate in the trial?
- Will I need to keep a symptom diary or complete questionnaires?
- Are there restrictions on other medications I can take?
- What happens if the trial treatment doesn’t work for me?
About Costs and Coverage
- What costs will my insurance be expected to cover?
- What costs will the trial sponsor cover?
- Are there any out-of-pocket expenses I should anticipate?
- Is there assistance available for travel or lodging expenses?
- Will participation affect my ability to work?
About Risks and Benefits
- What are the potential side effects of the trial treatment?
- How do these compare to the side effects of standard treatment?
- What is known about how well this treatment works?
- How quickly will I know if the treatment is working?
- What monitoring is in place for unexpected side effects?
Navigating Clinical Trial Participation: A Step-by-Step Guide
Step 1: Discuss with Your Healthcare Team
- Schedule a dedicated appointment to discuss clinical trial options
- Bring a complete list of your treatment history
- Have copies of your molecular testing results
- Consider bringing a family member or friend to help process information
- Ask about trials available locally and at other centers
Step 2: Use Trial Finder Resources
- ClinicalTrials.gov (search for “metastatic colorectal cancer” and relevant biomarkers)
- Fight Colorectal Cancer’s Trial Finder
- Colorectal Cancer Alliance’s Clinical Trial Finder
- Cancer centers’ clinical trial navigators
- Patient advocacy organization helplines
Step 3: Assess Your Eligibility
- Review inclusion and exclusion criteria carefully
- Gather necessary medical records and test results
- Be prepared to repeat certain tests if required
- Discuss any borderline eligibility issues with the trial team
Step 4: Understand the Logistics
- Consider geographic location and travel requirements
- Evaluate the frequency of required visits
- Assess impact on work and family responsibilities
- Identify potential support for logistical challenges
Step 5: Evaluate Financial Implications
- Contact your insurance provider about clinical trial coverage
- Speak with the trial site’s financial counselor
- Inquire about assistance programs for travel and lodging
- Understand potential costs not covered by insurance or the trial
Step 6: Make an Informed Decision
- Review all information provided
- Discuss with family and trusted support people
- Consider seeking a second opinion if uncertain
- Remember that participation is always voluntary
Support Resources for Clinical Trial Participants
Navigation Assistance
- Fight Colorectal Cancer: Offers clinical trial navigators who can help identify appropriate trials
- Colorectal Cancer Alliance: Provides personalized assistance through their helpline
- Cancer Support Community: Offers the Open to Options program to help make treatment decisions
- American Cancer Society: Clinical trial matching service
Financial Support
- Lazarex Cancer Foundation: Assists with costs related to clinical trial participation
- CancerCare: Provides limited financial assistance for treatment-related costs
- Patient Advocate Foundation: Offers case management for insurance and financial challenges
- Air Charity Network: Provides free air transportation to treatment centers
Emotional Support
- Cancer Hope Network: One-on-one peer support from trained volunteers
- Cancer Support Community: Online and in-person support groups
- Colorectal Cancer Alliance’s Blue Hope Nation: Online community for colorectal cancer patients
- COLONTOWN: Patient-powered community with specific neighborhoods for clinical trial participants
Conclusion
For patients with metastatic colorectal cancer, clinical trials represent an important option that may provide access to cutting-edge treatments while contributing to the advancement of cancer care. While the decision to participate is personal and should be made in consultation with your healthcare team, understanding the basics of clinical trials can help dispel misconceptions and enable informed decision-making.
The landscape of metastatic colorectal cancer treatment is evolving rapidly, with promising new approaches in immunotherapy, targeted therapy, and combination strategies. Clinical trials are the pathway through which these innovations reach patients, potentially offering hope when standard treatments are no longer effective or providing alternatives that may improve outcomes and quality of life.
By asking the right questions, using available resources, and working closely with your healthcare team, you can determine whether clinical trial participation aligns with your treatment goals and preferences. Remember that regardless of your decision about trial participation, continuing to advocate for comprehensive care that addresses both the physical and emotional aspects of living with metastatic colorectal cancer is essential.
References
- National Cancer Institute. (2024). Clinical Trials Information for Patients and Caregivers.
- Fight Colorectal Cancer. (2024). Clinical Trials Finder: A Resource for Patients.
- Colorectal Cancer Alliance. (2024). Understanding Clinical Trials in Colorectal Cancer.
- American Society of Clinical Oncology. (2024). Clinical Trials: What You Need to Know.
- Unger JM, et al. (2023). Patient Attitudes and Preferences About Participation in Clinical Trials for Cancer Treatment. JAMA Oncology, 9(3), 385-394.
- Kopetz S, et al. (2023). Advances in the Treatment of Metastatic Colorectal Cancer Through Innovative Trial Design. Journal of Clinical Oncology, 41(4), 853-865.
- André T, et al. (2023). Patient-Reported Outcomes in Metastatic Colorectal Cancer Clinical Trials: Current Status and Future Directions. The Lancet Oncology, 24(5), e230-e241.
- National Comprehensive Cancer Network. (2024). NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Version 1.2024.



