Book Appointment Now
Astrocytoma Survival Rates and Prognosis: What Patients Need to Know
If you or a loved one has been diagnosed with astrocytoma, understanding survival rates, life expectancy, and prognosis can feel overwhelming. This article answers critical questions like, “How long can someone live with astrocytoma?” and “What factors influence outcomes?” We’ll explore the latest data, clarify key terms, and explain how modern treatments are reshaping survival trajectories.

Astrocytoma Survival Rates: Key Statistics
Survival rates depend on tumor grade, molecular markers, age, and treatment access. Below are 2024-updated statistics:
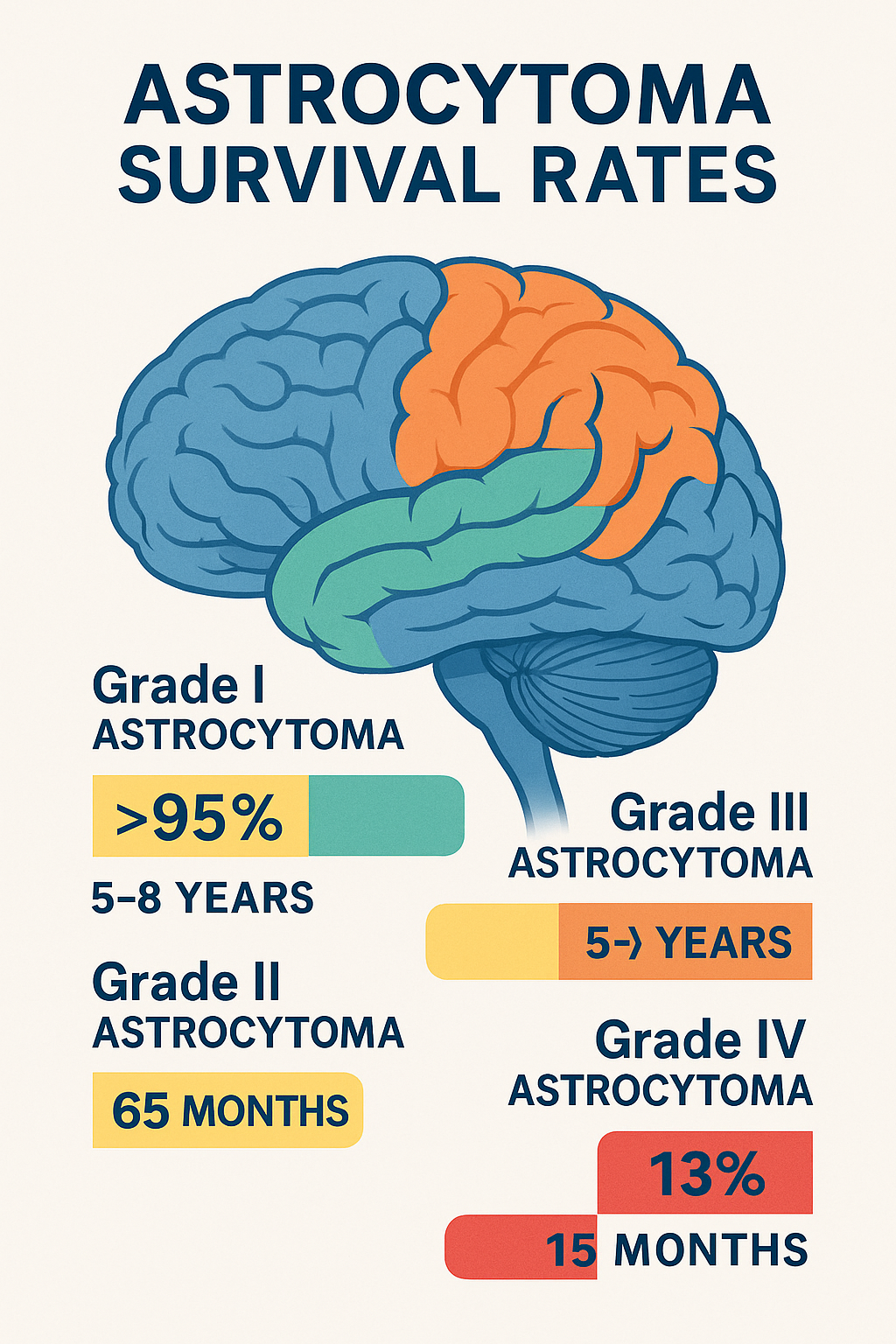
Grade I (Pilocytic Astrocytoma)
- 10-year survival: >95% after complete surgical removal.
- 5-year survival: 97% in children (2022 study).
- Key Insight: Rarely progresses to higher grades; recurrence is uncommon with total resection.
Grade II (Diffuse Astrocytoma)
- Median survival:
- IDH-mutant: 9.3 years (combined Grades II-III).
- IDH-wildtype: 5-8 years.
- 5-year survival: 73% for ages 20-44 vs. 46% for 45-54.
- Breakthrough: Vorasidenib (FDA-approved 2024) doubled progression-free survival (27.7 vs. 11.1 months) in IDH-mutant tumors.
Grade III (Anaplastic Astrocytoma)
- Median survival:
- IDH-mutant: 65 months (5.4 years).
- IDH-wildtype: 20 months (1.6 years).
- 3-year survival: 71% for young patients (<40) with total resection.
Grade IV (Glioblastoma)
- Long-term survivors: 5% live >5 years.
- Median survival:
- Standard therapy (TMZ + radiation): 15 months.
- TTFields + TMZ: 21 months (5-year survival: 13% vs. 5%).

5 Prognostic Factors Shaping Outcomes
- IDH Mutation Status:
- 80% of low-grade astrocytomas have IDH mutations, linked to 2-3x longer survival.
- Vorasidenib, an oral IDH inhibitor, reduces progression risk by 61%.
- MGMT Promoter Methylation:
- Present in 40-50% of glioblastomas; improves TMZ response (23 vs. 15 months survival).
- Surgical Resection:
- Gross total resection improves survival by 30-50% across all grades.
- Age:
- Patients <50 have 2-3x better survival in high-grade tumors.
- Emerging Therapies:
- Tumor Treating Fields (TTFields) and personalized vaccines are changing outcomes.
How Treatment Impacts Survival
1. Surgery
- Maximal safe resection is critical. Even partial removal enhances radiation/chemotherapy efficacy.
2. Radiation + Chemotherapy
- Standard for Grades III-IV: TMZ + radiation improves survival by 5-9 months.
- New Protocol: Pembrolizumab (immunotherapy) + TMZ is under investigation.
3. Tumor Treating Fields (TTFields)
- Mechanism: Wearable device disrupting cancer cell division.
- Results: TTFields + TMZ extend median survival to 21 months (vs. 16 months with TMZ alone).
4. Targeted Therapies
- Vorasidenib: First FDA-approved IDH inhibitor for low-grade gliomas (2024).
- Ivosidenib: Approved for IDH-mutant glioblastoma.
5. Clinical Trials
- Liquid biopsies: Detect tumor DNA in blood/CSF to monitor recurrence.
- Personalized vaccines: Phase 1 trials show immune responses in glioblastoma.
Living Well with Astrocytoma: Practical Strategies

- Specialized Care: Seek neuro-oncologists at academic centers (e.g., MD Anderson, Mayo Clinic).
- Rehabilitation: Physical/cognitive therapy mitigates fatigue and memory loss.
- Support Networks: National Brain Tumor Society offers clinical trial navigation and financial aid.
The Future of Astrocytoma Care
Safety Features:
- Liquid Biopsies: Replace invasive biopsies for monitoring recurrence (90% accuracy in trials).
- Combination Therapies: Vorasidenib + pembrolizumab trials show 40% tumor reduction.
- AI-Driven Treatment: Algorithms predict optimal drug combinations based on tumor genetics.
Key Takeaways
- IDH mutations and MGMT methylation are critical for prognosis.
- TTFields and vorasidenib represent major advances.
- 5-year survival ranges from >95% (Grade I) to 13% (Grade IV with TTFields).
Next Steps for Patients
- Ask your care team:
- “What is my tumor’s molecular profile (IDH, MGMT)?”
- “Am I eligible for TTFields or vorasidenib?”
- “Are clinical trials for liquid biopsies or vaccines available?”
References
- Louis DN et al. 2021 WHO CNS Tumor Classification. Acta Neuropathol. 2021.
- Mellinghoff IK et al. Vorasidenib in IDH-Mutant Low-Grade Glioma. NEJM. 2023.
- Stupp R et al. TTFields + TMZ for Glioblastoma. JAMA. 2017.
- Miller AM et al. Liquid Biopsies in Glioma. Nature. 2019.
Disclaimer: This article is for educational purposes. Treatment plans must be tailored by a neuro-oncology team.
For more information on cancer care and advancements in oncology equipment, visit CancersHub.com.



